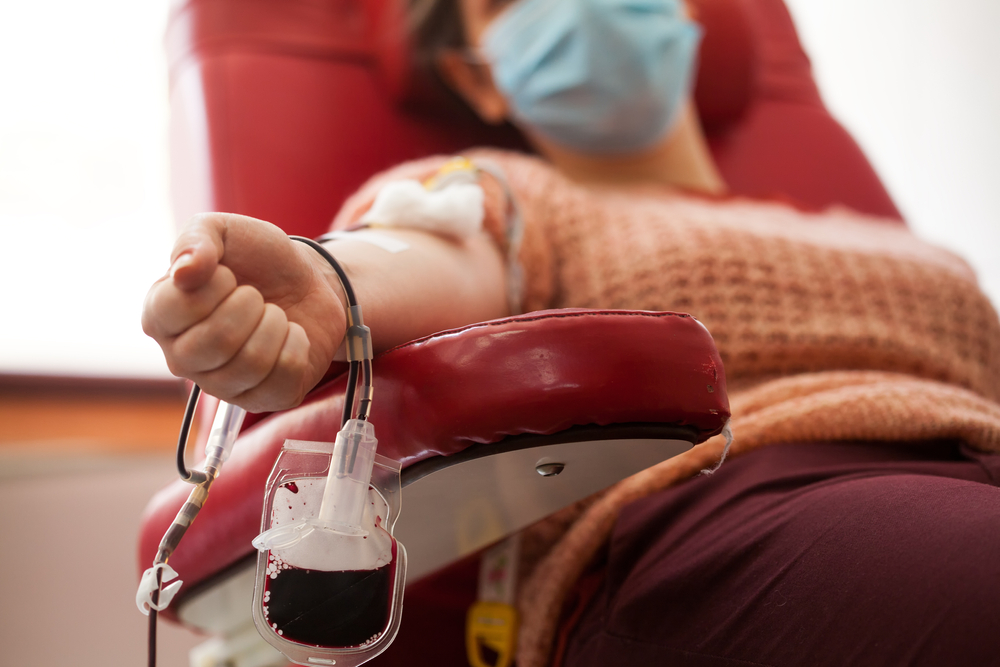
The Biomedical Advanced Research and Development Authority (BARDA) is collaborating with the Mayo Clinic on an expanded access program that will get patients hospitalized with COVID-19 access to convalescent plasma.
Convalescent plasma’s use is built on the idea that those who recover from infections are likely to develop antibodies to those infections. While the efficacy of this for COVID-19 remains uncertain, plasma containing antibodies — plasma collected from recovered patients — has the potential to bind a targeted virus and neutralize it in those still suffering from the disease. On the whole, it also has the advantage of being much more straightforward than developing a new therapeutic or vaccine.
With an expanded access program, Mayo will be able to use such plasma outside of a clinical trial setting. The clinic also served as the central Institutional Review Board for overseeing the expanded access program. That program enrolls patients from hospitals across the country.




