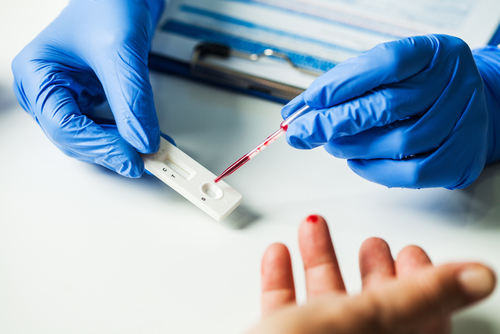
The Assure COVID-19 IgG/IgM Rapid Test Device this week became the first antibody point-of-care test for COVID-19 to receive emergency use authorization from the U.S. Food and Drug Administration (FDA).
Originally granted authorization for use by certain labs in July, the new EUA allows the test to be utilized with fingerstick blood samples capable of being tested in settings such as doctors’ offices, hospitals, and urgent care centers. Lab testing requirements have been removed, and the test can be used with venous whole blood, serum, plasma, and fingerstick whole blood.
“Authorizing point-of-care serology tests will enable more timely and convenient results for individuals who want to understand if they have previously been infected with the virus that causes COVID-19,” FDA Commissioner Stephen Hahn said. “Until today, serology test samples were generally only able to be evaluated in a central lab, which can be time-consuming and use additional resources to transport samples and run the test. As more and more point-of-care serology tests are authorized, they will help conserve those resources and may help reduce processing time for other types of COVID-19 tests, as less time is spent on serology tests.”
The Assure test is available by prescription only and does come with certain warnings. The FDA still notes that the results from such serology tests should not be taken as justification for halting protective measures for themselves and others. Even if negative, the tests do not mean that patients should cease social distancing or stop wearing masks.
Researchers are still uncertain how long SARS-CoV-2 — the virus that causes COVID-19 — antibodies remain following infection, nor do they know for certain if these antibodies provide protective immunity. Further, these tests only detect antibodies — they do not identify the virus itself. This can produce false results.




