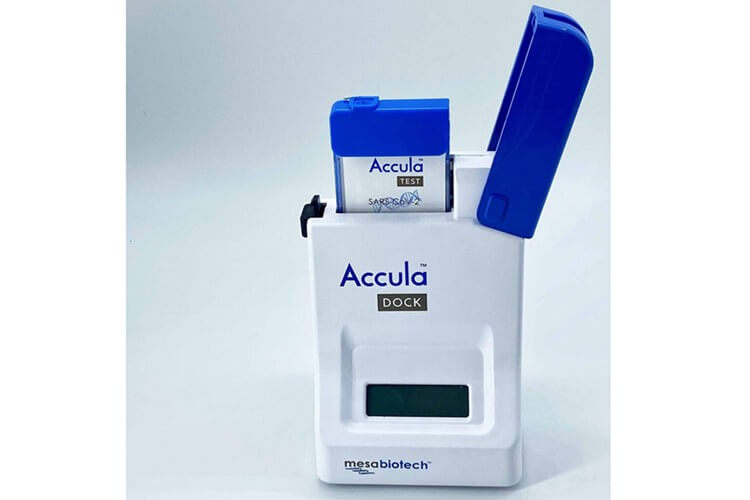
The Biomedical Advanced Research and Development Authority (BARDA) announced last week that it will collaborate with Mesa Biotech, Inc. on its rapid combination test meant to diagnose and differentiate between SARS-CoV-2 – the virus behind COVID-19 – and influenza.
Known as Accula, the test will use the same instrument as Mesa’s existing Flu A/Fly B test and SARS-CoV-2 test. It will be capable of turning results around in approximately 30 minutes. By combining these tests into one item, BARDA and Mesa believe health care providers will benefit, since it could potentially allow them to distinguish from SARS-CoV-2 cases with throat, nasal or nasopharyngeal swab samples.
With flu season looming and COVID-19 cases rising, the notions of facilitating proper treatment and reducing stress on an already stressed medical infrastructure, also factor in heavily. The Accula Flu A/Flu B test was already cleared by the U.S. Food and Drug Administration. Separately, development of the SARS-CoV-2 test began receiving BARDA support in March and eventually gained an Emergency Use Authorization.
Both existing tests are currently Clinical Laboratory Improvement Amendments (CLIA) waived for use in point of care operations. For the combined test, BARDA will support development efforts needed to request another Emergency Use Authorization and to gain 510(k) clearance.




