Through a new contract announced this week, the Biomedical Advanced Research and Development Authority (BARDA) will provide up to $30 million to Invirsa, a small Columbus, Ohio, biopharmaceutical company, for a treatment that could hold the cure to DNA-damage from sulfur mustard injuries in chemical warfare.
The drug is known as INV-102, and Invirsa believes it could be the means of treating ocular conditions caused by sulfur mustard and also other DNA damage-causing diseases, Invirsa CEO Dr. Bob Shalwitz told Homeland Preparedness News in an interview that touched on the new contract and why a cure for a weapon used as far back as WWI is still important today.
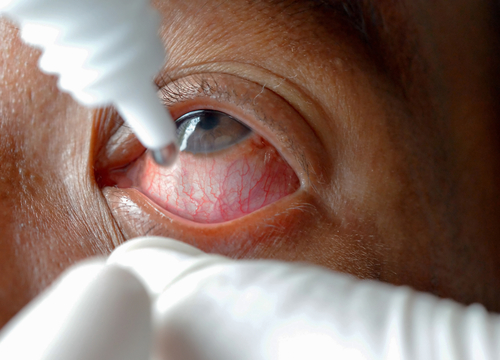
Sulfur mustard is a type of chemical warfare agent and irritant that absorbs into skin and mucous membranes, causing them to blister on contact, damaging the skin, eyes and respiratory tract. It’s not that difficult to manufacture, yet can completely deactivate its victims. Pain and swelling can eventually turn to permanent blinding of the eyes, an effect thought to be caused primarily by DNA damage. In this, it shares certain traits with the more common eye condition pink eye, which also causes DNA damage.
“It’s the archetypal DNA injury,” Shalwitz said. “It not only causes initial irritation that starts a few hours after exposure, it causes severe swelling of the front of the eye, the cornea, that leads to obstruction. You need something that alters that healing process. The science had to catch up: could you really do something about DNA injury? That’s the fundamental part of healing.”
DNA injuries are fairly common. According to Shalwitz, a typical cell undergoes a constant repair process, but things like radiation injuries, X-ray injuries, chemical injuries and viral injuries add up.
No drugs approved by the Food and Drug Administration (FDA) to treat sulfur mustard ocular injuries currently exist. A treatment has been sought for more than a century, but unlike past attempts, the data from INV-102 has been promising. Derived from naturally occurring molecules, it works through something as simple as an eye drop. INV-102 stimulates the body’s repair process by stimulating proteins.
While Invirsa couldn’t conduct a study on sulfur mustard in humans due to obvious ethical issues, the company has pursued a broad indication that will include conditions associated with damage in the eye, including viral or other forms of injury, with pink eye being a major focus. An additional indication will allow it to examine ultraviolet rays and the DNA damage they cause in the eye. BARDA will provide some technical support in addition to funding, but Invirsa will stay at the helm of the project, even in seeking approval with the FDA.
“This is my first big federal contract,” Shalwitz said. Shalwitz is also a pediatric endocrinologist, with extensive background in clinical research. He has previously taken two pharmaceutical startups to public offerings, and led teams to new and supplemental drug approvals at Abbott Laboratories and Reliant Pharmaceuticals. “To get the approval, we already identified the groups we would work with around the U.S. and Canada to handle manufacturing all the way to clinical trials.”
Under the terms of the arrangement, BARDA will provide a base period of approximately $14.4 million to Invirsa, which will cover preclinical, Phase One and some Phase Two work. After passing certain hurdles and progressing to Phase Three, the company could receive an additional $15.2 million in funding.
Shalwitz said he was grateful that BARDA was willing to move forward with the contract, even in the midst of the collective push for a COVID-19 vaccine, which has captivated much of the scientific focus throughout the country.

