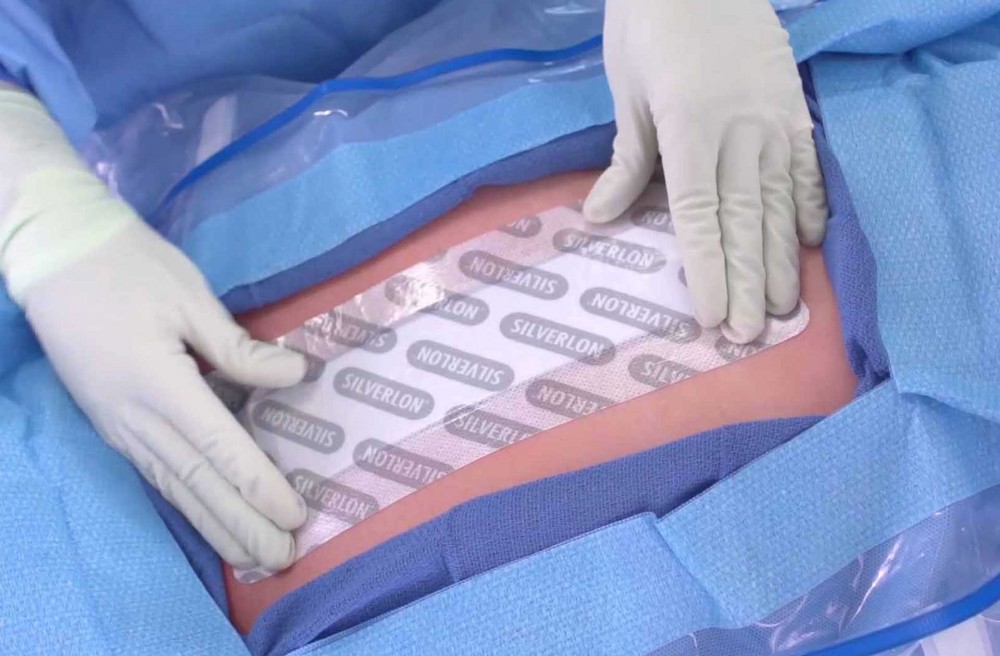
The U.S. Food and Drug Administration recently cleared the first use of a commercially available product to manage blister injuries caused by sulfur mustard, otherwise known as mustard gas.
Silverlon, a silver-plated nylon dressing, is used widely to aid in the management of acute skin wounds and first- and second-degree thermal burns. The silver plating helps kill bacteria within the dressing, and one dressing can be used for up to seven days. This allows for fewer dressing changes, which reduces the burden on caregivers and minimizes the pain and damage that would occur if the wound was disturbed, according to a press release issued last week by the U.S. Department of Health and Human Services.
“Our top priority is saving lives during national emergencies. To do so, we must make safe and effective medical products for all the illnesses and injuries stemming from the serious health security threats confronting our nation,” said Robert Kadlec, Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services. “This product clearance is the latest step in delivering on that promise to the American people.”
Argentum Medical has received FDA clearances for multiple indications for Silverlon since 2003 and in that time the wound dressing has been used extensively by the U.S. military to treat burn and blast wounds. Silverlon dressings also are used widely by the healthcare and first responder communities.
The ASPR’s Biomedical Advanced Research and Development Authority (BARDA) provided technical expertise and funding to support the studies necessary to show that the product is appropriate for use on first- and second-degree skin burns caused by exposure to sulfur mustard.
“Chemical weapons like sulfur mustard cause horrific, painful, and life-altering injuries, yet in the 100-year history of sulfur mustard use, no medical countermeasures existed – until now,” said Rick Bright, director of BARDA. “At BARDA, we are excited to have supported the first cleared product for use on skin injuries caused by sulfur mustard. This clearance exemplifies BARDA’s ongoing commitment to our partners and the nation as we seek out promising technologies and products to improve our nation’s health security and protect Americans.”
BARDA’s support for this additional indication began in 2013 as part of the federal government’s effort to repurpose approved drugs and medical products to save lives and reduce injury in an attack on the United States. This multi-purpose approach has proven to be cost effective in preparing mass casualty emergencies from chemical, biological, and radiological agents. Repurposing products in widespread use additionally ensures first responders have a familiar product to use in a time of crisis.
BARDA used Project BioShield authorities and the Project BioShield Special Reserve Fund to purchase Silverlon for the Strategic National Stockpile as part of BARDA’s burn countermeasure program. In 2004, Congress passed the Project BioShield Act to create a market for products necessary for disaster response but with limited or no commercial market. The Act provides HHS with a multi-year special reserve fund to support late-stage development and manufacturing, and the financial resources to buy life-saving medical products for the American people to use in public health emergencies, Kadlec wrote earlier this month in a blog post.
“In this way, Project BioShield is a critical part of the U.S. strategy for biodefense and our commitment to the American people,” Kadlec wrote.

