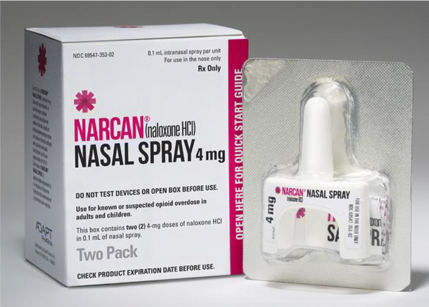
The U.S. Food and Drug Administration (FDA) granted last week its final approval of Narcan, a medication designed to stop or reverse the effects of an opioid overdose.
Narcan, developed by Teva Pharmaceuticals, is the first generic naloxone hydrochloride nasal spray.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible. In addition to this approval of the first generic naloxone nasal spray, moving forward we will prioritize our review of generic drug applications for naloxone,” Douglas Throckmorton, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said. “All together, these efforts have the potential to put a vital tool for combatting opioid overdose in the hands of those who need it most – friends and families of opioid users, as well as first responders and community-based organizations. We’re taking many steps to improve availability of naloxone products, and we’re committed to working with other federal, state and local officials as well as health care providers, patients, and communities across the country to combat the staggering human and economic toll created by opioid abuse and addiction.”
While this is the first naloxone nasal spray, generic injectable naloxone products have been available for years. While business and other considerations may impact how quickly this product becomes available, this approval is an important step toward expanding access to this drug.
Almost 400,000 people have died from an opioid overdose from 1999 to 2017, according to the Centers for Disease Control and Prevention. On average, more than 130 Americans die every day from overdoses including opioids and illegal drugs.
As part of the U.S. Department of Health and Human Services’ ongoing efforts to combat the opioid crisis, the FDA is working to increase access to generic naloxone products.




