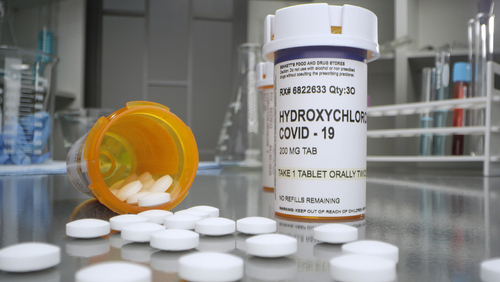
Arguing the potential benefits no longer outweigh the health risks involved, the U.S. Food and Drug Administration (FDA) on Monday revoked its Emergency Use Authorization for hydroxychloroquine and chloroquine to be used as a treatment for patients with COVID-19.
“We’ve made clear throughout the public health emergency that our actions will be guided by science and that our decisions may evolve as we learn more about the SARS-CoV-2 virus, review the latest data, and consider the balance of risks versus benefits of treatments for COVID-19,” Dr. Anand Shah, FDA Deputy Commissioner for Medical and Scientific Affairs, said.
The FDA made the decision in the wake of its own ongoing analysis, as well as emerging scientific data, which showed neither drug would likely be effective in treating the disease. Neither showed any benefit to mortality rates or in speeding recovery in a recent large randomized clinical trial of hospitalized patients. Other studies have consistently shown a lack of benefit to either drug. Further, those drugs elicited serious heart problems and other serious side effects.
The Biomedical Advanced Research and Development Authority (BARDA) within the U.S. Department of Health and Human Services originally requested the Emergency Use Authorization (EUA) covering chloroquine and hydroxychloroquine, and the FDA granted it on March 28. President Donald Trump
has frequently touted hydroxychloroquine as a treatment for COVID-19 despite a lack of scientific evidence that it was effective, and in fact said he had taken the drug himself as a preventative measure.
But today, in consultation with the FDA, BARDA sent a letter to the FDA requesting revocation of the EUA based on up-to-date science and data.
Although chloroquine phosphate and hydroxychloroquine sulfate have been donated to the Strategic National Stockpile for treatment of COVID-19 when clinical trials were either unavailable or unfeasible, they will no longer be used for that purpose. Both remain FDA approved for the treatment or prevention of malaria. Hydroxychloroquine is also approved to treat autoimmune conditions such as chronic discoid lupus erythematosus, systemic lupus erythematosus and rheumatoid arthritis.
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate. This action was taken following a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Dr. Patrizia Cavazzoni, acting director of the FDA’s Center for Drug Evaluation, said.
“We remain committed to using every tool at our disposal in collaboration with innovators and researchers to provide sick patients timely access to appropriate new therapies. Our decisions will always be based on objective and rigorous evaluation of the scientific data,” Cavazzoni said.




