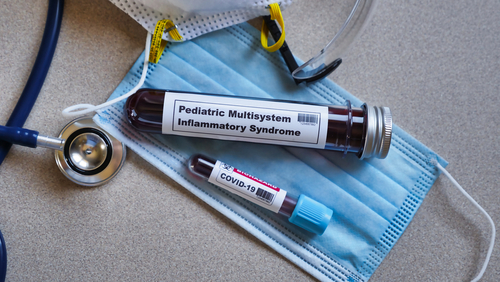
The Biomedical Advanced Research and Development Authority (BARDA) and Beckman Coulter are joining forces to fund a multi-center clinical trial of Beckman Coulter’s Monocyte Distribution Width (MDW) hematology biomarker, a test meant to rapidly detect MIS-C.
MIS-C, or Multisystem Inflammatory Syndrome in Children, is a rare but dangerous post-COVID-19 condition that has complicated recovery among more than 1,000 children in the U.S. Untreated, it can cause hypotensive shock, cardiac aneurysms, or ventricular failure — and that’s just in the short term. It’s too early to know its long-term effects. It’s also hard to pin down since its initial symptoms are merely mild things like fevers or rashes.
Worse, there are currently no tests for MIS-C, and cases could increase now that more children are crowded together again for school.
Enter: Beckman Coulter. Beckman Coulter’s MDW biomarker has already been cleared by the Food and Drug Administration (FDA) as a marker of sepsis among adults. It’s already used in emergency departments, and preliminary results from a Massachusetts General Hospital study have shown that it could assist in the rapid detection of MIS-C as well.
The new effort will be collaborative, and beyond Beckman Coulter, will include partners at Massachusetts General Hospital, Johns Hopkins University School of Medicine, and the University of Florida. If a trial proves successful, they will seek regulatory approval for the biomarker’s use on MIS-C.




