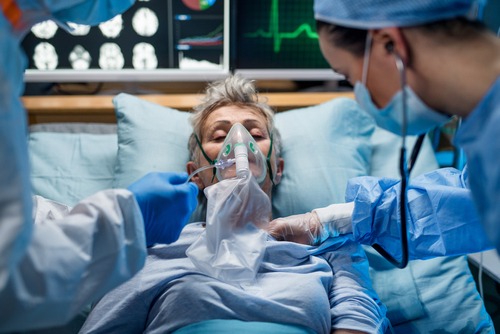
The Biomedical Advanced Research and Development Authority (BARDA) will provide $706,534 in funding for Dascena, Inc. to further its COViage system, potentially expanding its machine learning capabilities to predict deteriorating health among hospitalized COVID-19 patients.
Hospitalized COVID-19 patients are at serious risk of rapid health deterioration, but predicting it can be tricky, especially since few scoring programs exist to help, according to BARDA. COViage adds a certain layer of precision to these efforts, analyzing data from the patient’s electronic health record to advise clinicians of any heightened risks for unstable blood pressure or respiratory failure.
Ideally, medical staff could then intervene in time, such as mechanical ventilation, to prevent a crisis.
COViage has already received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) to predict unstable blood pressure and respiratory failure resulting in ventilation for COVID-19 patients. Clinical tests and user evaluations are planned to better assess and evolve the tool for greater use nationwide among clinicians.
Under the terms of this public-private partnership, Dascena will advance the system through the clinical validation process and ultimately push a request for FDA 510(k) clearance of COViage. User assessments will also be undertaken to promote implementation at additional clinical sites. The company will also pursue a randomized controlled trial to assess COViage’s algorithmic capabilities for predicting respiratory decline.




