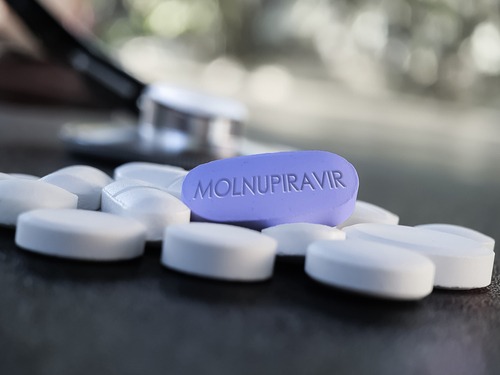
The United States federal government exercised two options on contracts with Merck and Ridgeback Biotherapeutics this week to purchase an additional 1.4 million courses of the investigational oral antiviral molnupiravir for use against COVID-19.
This deal will amount to approximately $1 billion if the medicine is granted Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA). This would bring the government’s total purchase of the drug to $2.2 billion and 3.1 million courses, all dedicated to treating mild to moderate cases of COVID-19 among at-risk adults. If it desires, it still has options to purchase another 2 million courses as well.
“We’re honored that the U.S. government has chosen to purchase more than 3 million courses of molnupiravir, our promising oral antiviral, so that molnupiravir, if authorized, will be among the vaccines and medicines available to fight COVID-19 as part of our collective efforts to bring this pandemic to an end,” Frank Clyburn, president of Human Health at Merck, said. “In light of the continued impact of the pandemic on hundreds of thousands of people every day, all of us at Merck are moving with urgency and rigor to bring molnupiravir, with its compelling data showing a significant reduction in death and hospitalizations, to patients as quickly as we can.”
An EUA application has already been filed with the FDA, specifically for use on those adult patients at risk of advancing to severe or hospitalized cases of COVID-19. The FDA’s Drugs Advisory Committee will address that application on Nov. 30, 2021.




