Vaccination has begun for a Phase 2/3 trial of Bharat Biotech International Ltd’s (BBIL) Chikungunya vaccine candidate, through a partnership with the International Vaccine Institute (IVI) in Costa Rica.
The Costa Rican trial of BBV87, the official name for the candidate, marks the beginnings of a multi-country study funded by the Coalition for Epidemic Preparedness Innovations (CEPI) and the Ind-CEPI mission of the Department of Biotechnology, India. This trial will be randomized and controlled, with a focus on safety and immunogenicity. Eventually, the partners hope to see the vaccine gain World Health Organization prequalification to enable its distribution through low and middle-income countries, as pushed by CEPI.
“Epidemic preparedness is a vital step in public health care. Bharat Biotech’s vaccine candidate is an ingenious, well-researched vaccine, and we thank the first volunteer from Costa Rica for participating in this study,” Dr. Krishna Ella, chairman and managing director of Bharat Biotech, said. “The IVI-led multi-country scale human trial has begun an important trial phase in furthering the evaluation of safety and immunogenicity. As a partner, we are committed towards GCCDP’s effort to realize a safe, efficacious vaccine that can help reduce Chikungunya disease burden world over.”
The Global Chikungunya vaccine Clinical Development Program, or GCCDP, endeavors to create and manufacture an affordable Chikungunya vaccine for distribution. As to IVI’s trial of BBV87, though, the candidate will eventually be distributed in a two dose regimen to healthy adults at nine clinical trial sites throughout five countries, including Costa Rica. Other participating nations will include Panama, Colombia, Thailand and Guatemala, which are all expected to begin studies this year.
“The start of this trial in Costa Rica is a significant milestone in the effort to make available a safe, effective, and affordable Chikungunya vaccine for the one billion people around the world at risk of Chikungunya virus infection,” Dr. Sushant Sahastrabuddhe, principal investigator and director of the GCCDP consortium, said.
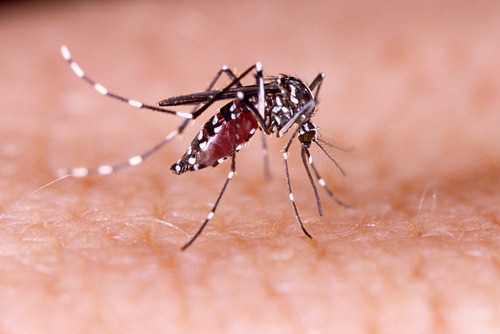
Chikungunya is spread by the bites of infected Aedes mosquitoes and causes fever, severe joint pain, muscle pain, headache, nausea, fatigue and rash. Since 2014, in the United States, local transmission of the virus has been reported in Florida, Puerto Rico, Texas and the U.S. Virgin Islands.
Since beginning support in June 2020, CEPI has provided up to $14.1 million for vaccine manufacturing and clinical development of BBV87. This was bolstered by a framework partnership agreement with the European Union and by another up to $2 million from the Ind-CEPI Initiative.




