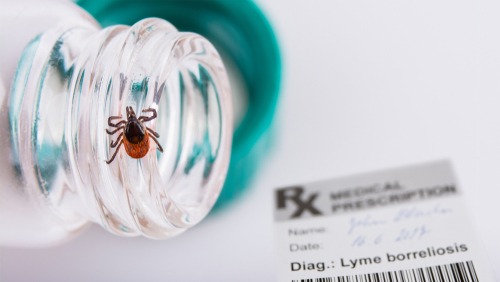
All 625 slots of a randomized Phase 2 trial of the joint-Valneva SE and Pfizer Inc. Lyme disease vaccine candidate VLA15-221 have been filled with participants, finishing the recruitment phase of the trial.
The trial is focused on participants between 5 to 65 years old. They will receive doses of either VLA15-221 or a placebo at monthly intervals, up to six months. For researchers, the main focus will be safety and immunogenicity data, which they hope will show down to its youngest participants, and then to use this data to evaluate the optimal vaccination schedule for use in a Phase 3 trial.
“This recruitment completion represents another important milestone in the development of VLA15,” Dr. Juan Carlos Jaramillo, Chief Medical Officer of Valneva, said. “If successful, this trial could enable the inclusion of a pediatric population in the Phase 3 trial. Lyme disease continues to be a major concern and is prevalent in children, it is therefore extremely important for us to potentially offer a vaccine that could protect both adults and children as rapidly as we can.”
The Phase 2 trial builds on the positive data received from two previous Phase 2 clinical trials of VLA15 in over 800 healthy adults. Significantly, the partners intend this latest to accelerate pediatric considerations for the vaccine candidate, which is already the only Lyme disease vaccine candidate under active clinical development currently.
“Given the medical importance of Lyme disease, its possible long term impact, and the known mechanism of vaccine protection, the development of a multivalent vaccine for prevention of 6 serotypes of Borrelia has the potential to address a great unmet need,” Dr. Kathrin Jansen, senior vice president and head of Pfizer Vaccine Research and Development, said. “We are pleased that the Phase 2 trial has reached full recruitment and look forward to what we hope will be a successful conclusion of the study.”
Based on current progress, topline results from this study are expected in the first half of 2022.




